The purpose of doing leaching tests is to obtain information on release under real field conditions. Gaining information under realistic field conditions is however not very practical in view of the amount of work and the cost involved. Besides, the number if situations to be evaluated makes this approach impossible. Leaching tests are therefore used to simulate the release as close as technically and practically feasible. A key aspect is the comparability of test results in case of dispute. For this purpose, leaching tests are standardised and the sensitivity of test results to limited variation in the test conditions evaluated through robustness testing. Intercomparison validation is subsequently carried out to determine test performance in terms of repeatability and reproducibility.
There are many leaching tests worldwide. Leaching tests can be described in a few general categories:
- Field simulation tests: Such tests can be set up at laboratory scale, but more frequently such tests are done under more realistic field exposure conditions on a manageable scale with collection of runoff and drainage water. Typically, lysimeters are used for this type of testing. As indicated this approach is relatively close to real practice, but also rather costly.
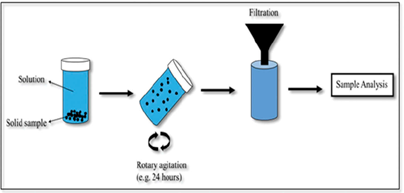
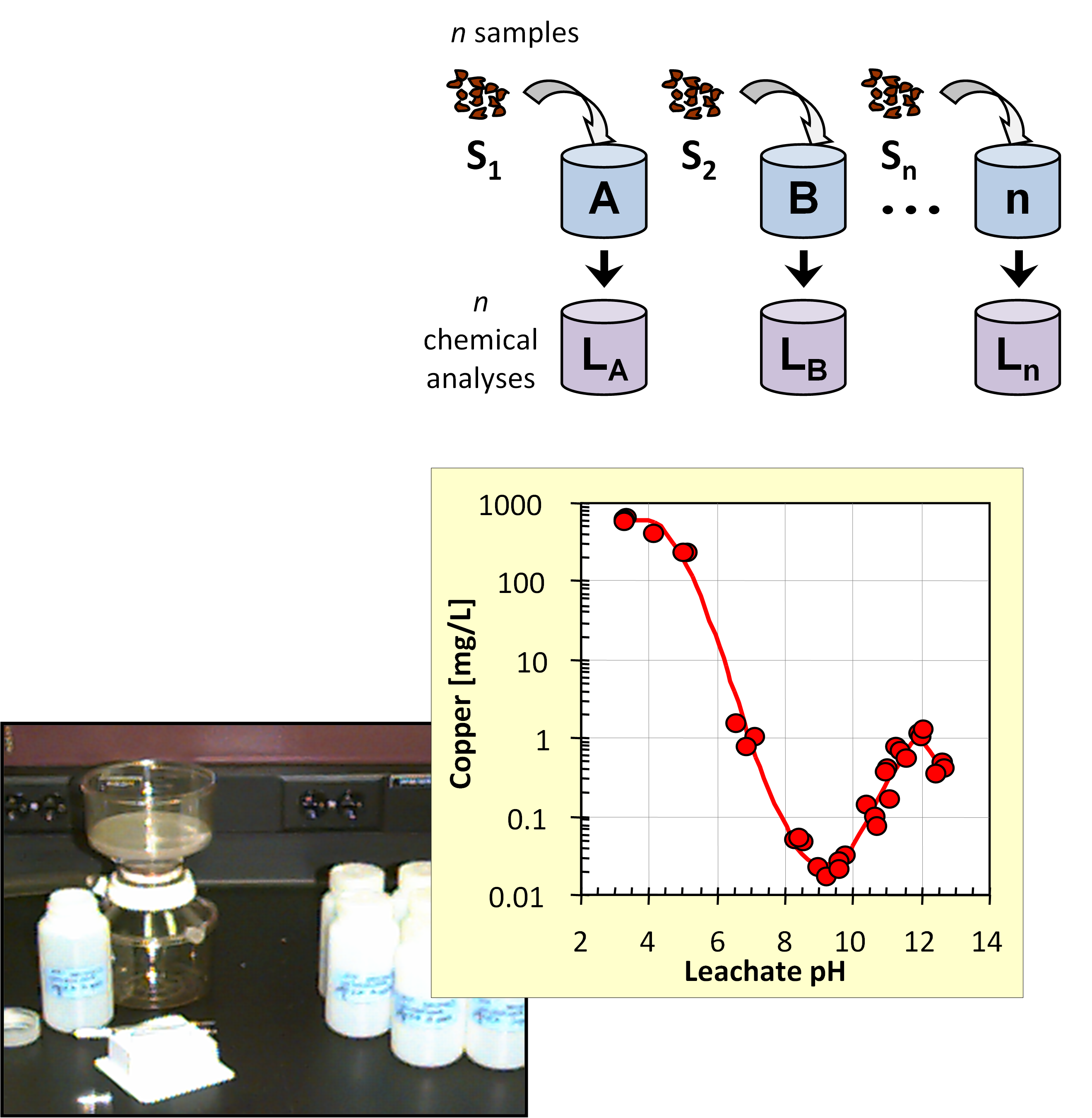
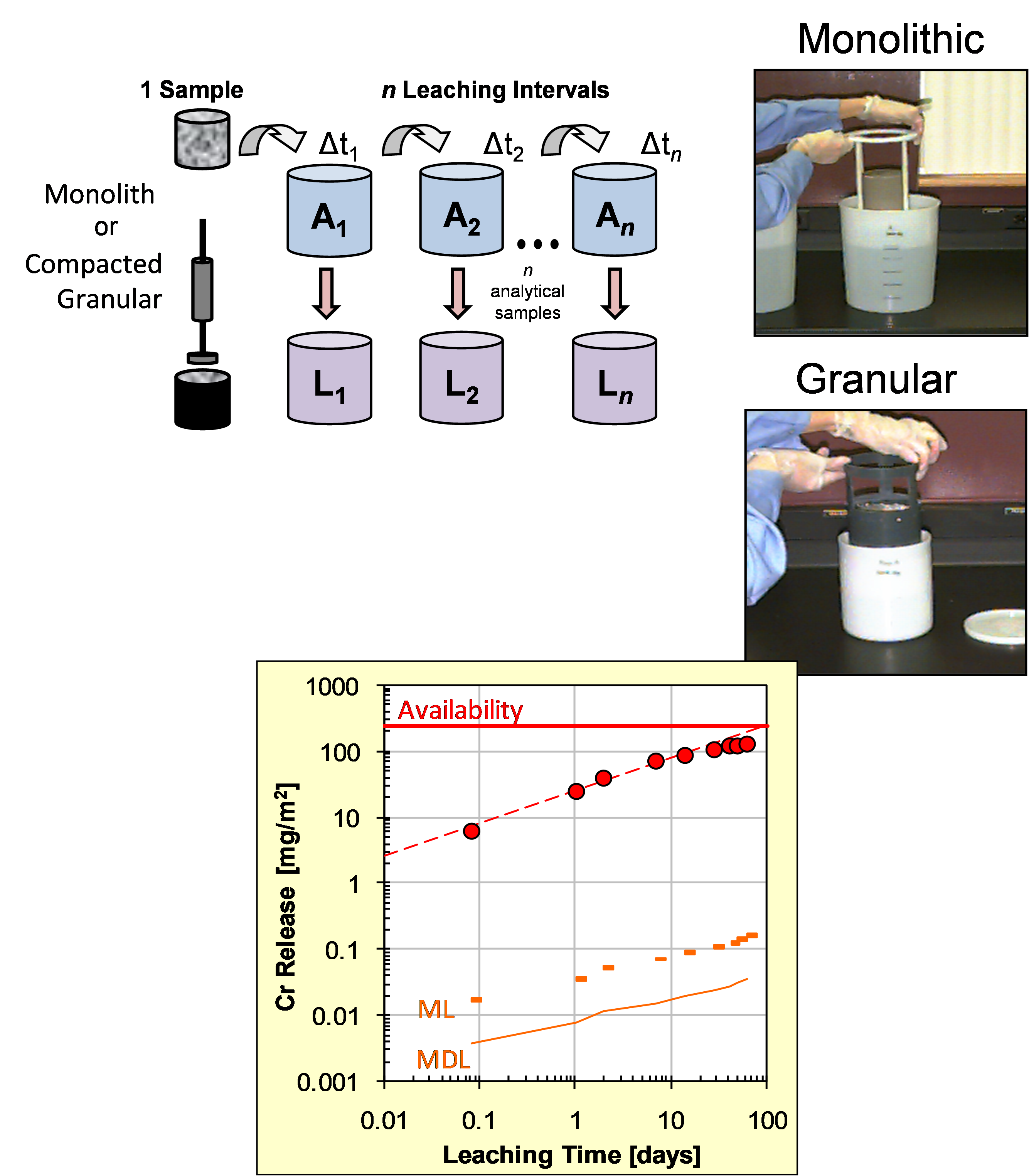
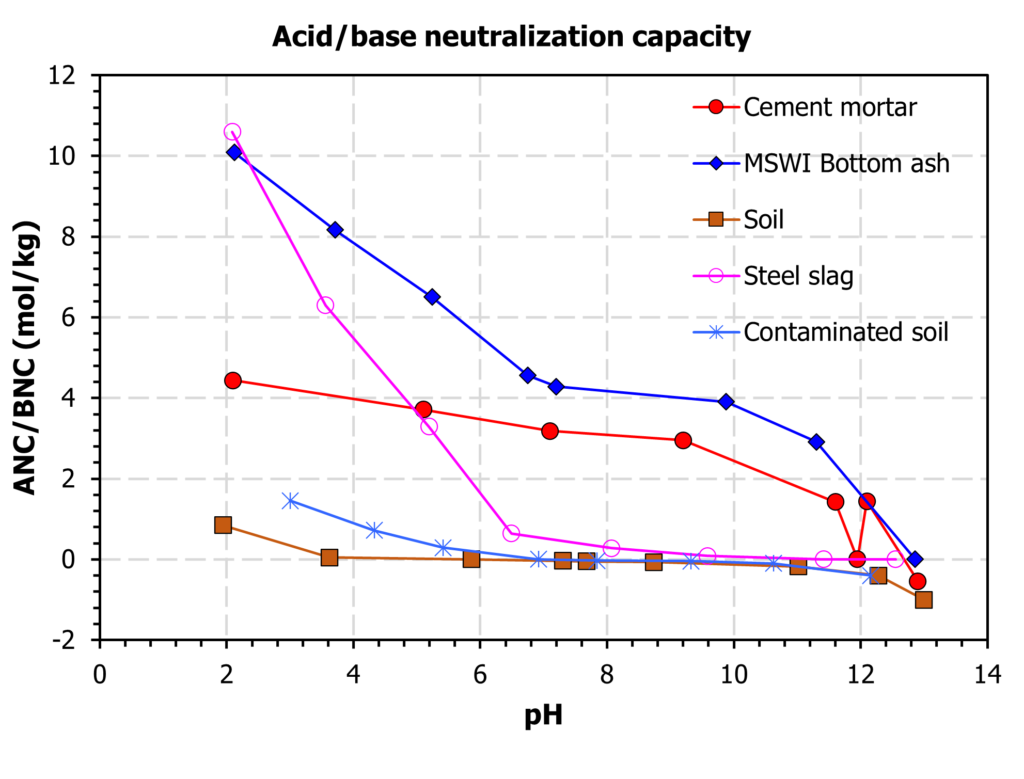
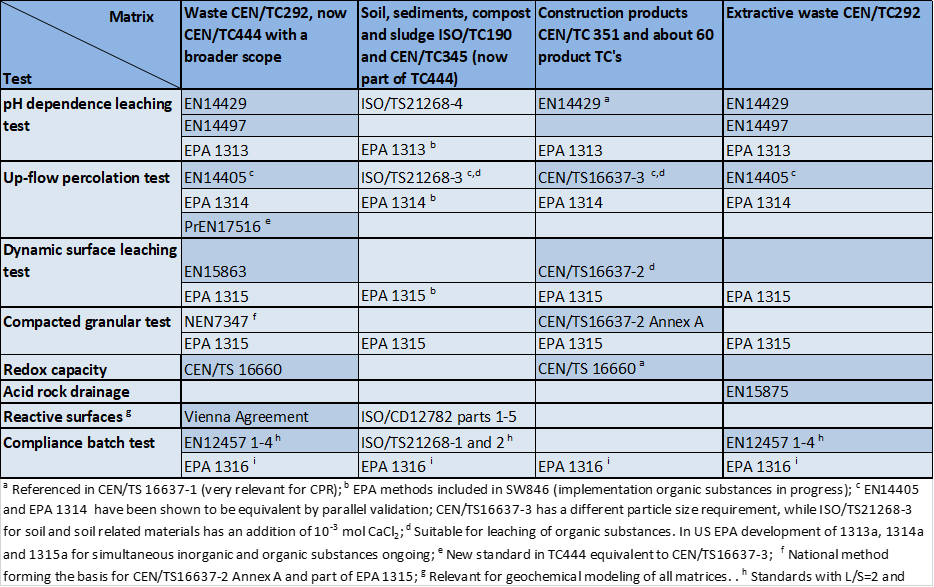
- Characterization: the tests establish the solid/liquid partitioning of inorganic or organic substances based on the physical nature of the waste or product and the degree of contact with the leachant or on imposed conditions such as controlling the pH to which the waste/product is exposed to assess the sensitivity to a change in pH conditions. The leachant is generally demineralized water. In case of granular materials, the release of substances is expressed as a function of liquid to solid ratio (L/S in L/kg). In case of a monolithic material, the release is expressed in mg/m2 as a function of time with a focus on diffusion-controlled release. More than one test is needed for full characterization to understand the different factors controlling release of substances in the short term and over extended time scales, when changes in release behaviour can be anticipated. Sub-categories within characterization are: percolation tests, monolith leach tests and pH dependent leaching tests.
- Compliance tests: simple tests to comply with regulatory criteria. Generally, the tests are single step batch leaching tests with a defined leachant. Examples are the EN 12457 series using demineralized water and US EPA Toxicity Characteristic Leaching Procedure (TCLP; based on acetic acid to simulate co-disposal with Municipal Solid Waste).
Due to the amount of work and cost involved the number of tests carried out deceases rapidly going from compliance testing to characterization testing to field simulation testing. Each level of test has its benefits and limitations. Verification of laboratory test results and predictions at field scale provide valuable insights in factors that cannot be mimicked in the laboratory. To solve a specific problem a tiered approach generally will prove very helpful.
More information on lab to field examples
Depending on the nature of the material inorganic substances or organic substances are of primary interest. Currently, inductively coupled plasma (ICP) methods either in combination with optical emission spectroscopy (OES) or mass spectrometry (MS) are mostly applied due to sensitivity and multi-element analysis capabilities. For organic substances, gas chromatography in combination with mass spectrometry is increasingly applied (GC-MS). In addition to these potentially critical substances, parameters such as pH, conductivity, redox state, presence of particulate and dissolved organic matter and reactive surfaces (hydrated metal oxides, clay) are important.