The inventory of potentially hazardous substances in solids can be determined by digestion and subsequent chemical analysis. However, the potential mobilisation of hazardous substances and their transfer to the environment by contact with water cannot be evaluated by solid matter analysis. Nowadays laboratory leaching tests are well established tools to assess a more realistic potential for impact under changing conditions of exposure.
Why are leaching data presented in logscale vs linear scale? In figure 1 the results of different leaching tests are displayed as a function of pH in context with the total content (complete digestion) and a few partial digestion methods. The green field in the left graph shows the normal pH range in sediment with at least an order of magnitude difference in leached amount over that pH range. There are almost 3 orders of magnitude difference between the total content and the actual leaching level. There is almost a factor 4 difference between the total content and the available content (defined as the maximum leached amount over the pH range 1.5 – 13). This available content can already be considered extreme from an environmental impact perspective. Regulatory criteria for leaching are in the 0.5 mg/kg range, which makes a total content value a long shot. In this context, discussion about partial or total digestion is not very relevant with the exception of mass balance studies.
To answer the question: all detail of leaching behaviour is lost when data are displayed using a linear scale (see right graph in figure 1 below) and the relevant aspects of leaching are in the low release level rather than at total content level. From the graphs it is also obvious that there is no relation between leaching and content except for highly soluble elements like Na and Cl that are leachable under all exposure conditions.
More information on solubility vs washout
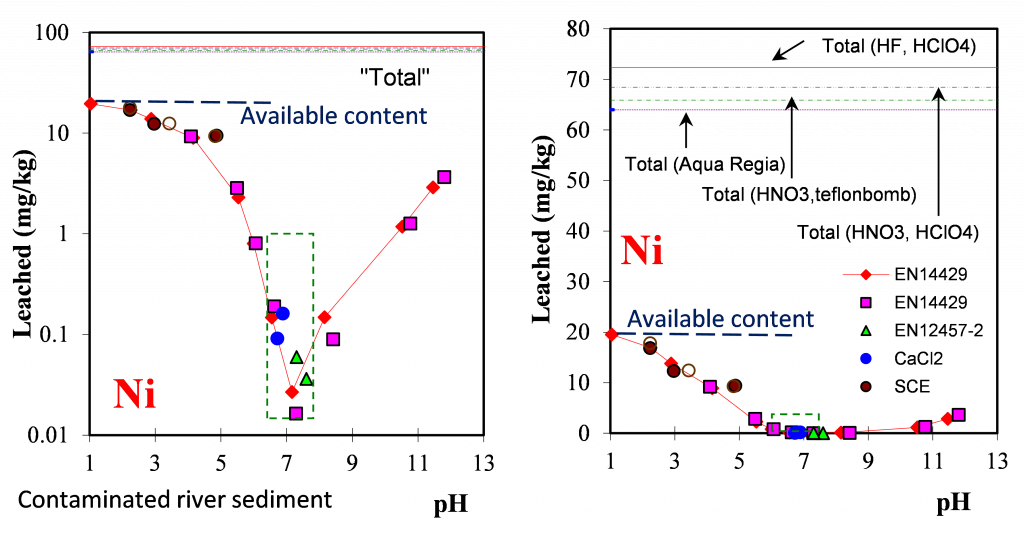
Figure 1. Ni leaching from contaminated river sediment as a function of pH to illustrate logscale vs linear data display.
The large gradients in leaching as a function of pH are the result of solubility control by minerals, sorption on hydrated iron-oxide or similar reactive metal-oxide surfaces, interaction with dissolved and particulate organic matter (humic-, fulvic acid type phases), binding to clay surfaces and incorporation in solid solution or any combination thereof.
Return to Homepage


